(1) Genomics on hearing loss

Hearing loss is the most common sensory disorder in humans, and its prevalence has been increasing worldwide over the past 20 years. According to the World Health Organization (WHO), there were approximately 460 million people with hearing loss globally in 2018. Congenital hearing loss is known to occur at a rate of about 2.5 per 1,000 newborns. Since 2015, our laboratory has been collaborating with Professors Jaeyoung Choi and Jinsei Jung from the Department of Otorhinolaryngology at Severance Hospital to establish a cohort of hearing loss patients (Yonsei University Hearing Loss, YUHL). Using DNA samples from these patients, we have conducted whole exome sequencing (WES) and whole genome sequencing (WGS) to provide molecular-level diagnoses and to pursue the following research objectives.
Overall goal: Elucidate novel genetics and pathobiology of hearing loss.
Specific aim 1: Discover novel single-gene causes of hearing loss in YUHL cohort and functionally characterize novel genes.
Specific aim 2: Elucidate the gene-gene interactions (oligogenic inheritance) that contribute to development of hearing loss.
Specific aim 3: Identify genes and pathways that contribute to noise-induces hearing loss.
Specific aim 4: Delineate the novel pathogenic mechanism of hearing loss and suggest therapeutic approaches.
Related publications Link
(2) Cancer metastasis and anchorage dependency of cell

In most cases, patients with advanced metastatic cancer are beyond curative treatment, and in fact, the vast majority of cancer-related deaths (>90%) are due to metastatic tumors rather than primary tumors. Despite this, there has been a significant imbalance in research, with many studies focusing on the mechanisms of primary tumor development while studies on metastatic cancer remain limited.
Our research team, in collaboration with Professor Hyun Woo Park’s lab in the Department of Biochemistry at Yonsei University, has identified a set of genes that regulate cellular adhesion, referred to as AST (Adherent-to-Suspension Transition) factors. We coined the term “AST” to describe the process by which adherent cells transition into suspension cells under the influence of these genes. During cancer metastasis via the bloodstream, tumor cells undergo a similar transformation into suspension cells, known as circulating tumor cells (CTCs).
Our laboratory is currently investigating the roles of AST factors in the formation of circulating tumor cells and their contribution to cancer metastasis. This research was supported by the National Science Challenge Initiative.
AST Metastasis Research Center homepage AST암전이연구단
Related publications Link
(3) Gentic kidney diseases and kidney organoids
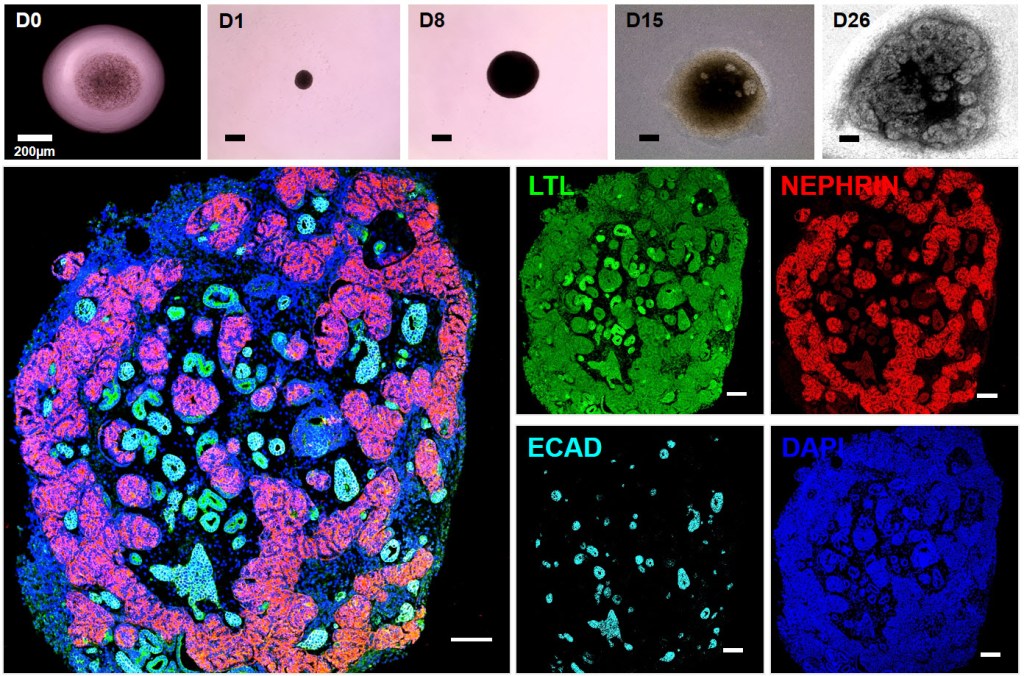
Our laboratory is conducting research on rare hereditary kidney diseases. We focus on genes associated with ciliopathies—disorders caused by abnormalities in the primary cilia of renal tubular cells—such as nephronophthisis, as well as genes involved in nephrotic syndrome, which results from abnormalities in podocytes of the glomerulus.
To investigate the functions and pathological mechanisms of these genes in the kidney, we utilize both mouse models and kidney organoids. Furthermore, we are developing a drug screening platform by integrating kidney organoid technology with single-cell transcriptomic analysis.
Related publications Link
(4) Other studies
1) Genome-wide assoication studies – we are conducting GWAS to identify genetic factors associated with various diseases, including non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), childhood asthma, and chronic kidney disease. Through GWAS, we aim to uncover disease-related genetic variants that contribute to the development and progression of these conditions.
Related publications Link
2) Motile ciliopathy – We are conducting research to elucidate the functions of genes responsible for primary ciliary dyskinesia (PCD), a disorder caused by abnormalities in motile cilia. Our studies aim to understand how defects in these genes disrupt the structure and function of motile cilia, ultimately leading to the pathogenesis of motile ciliopathy.
Related publications Link